Leprosy Mailing List – December 3, 2020
Ref.: (LML) Replacing opinions about HD treatment with evidence
From: Joel Almeida, Mumbai and London
Dear Pieter and colleagues,
Colleagues in endemic countries have drawn attention to some disturbing challenges. There is a neglected multitude of previously treated persons still requiring anti-microbial treatment. They are mostly anergic and smear positive. They bear the brunt of additional nerve damage, worsening visible deformity, exclusion and extreme poverty. Nobody likes to see human beings reduced to such a state. Further, anti-microbial neglect constrains those with anergy to produce astronomical numbers of concentrated viable bacilli. This is bad for the population and worse for the individuals affected.
Attention to evidence usually helps distil more effective action. We can keep discerning what works or does not, and keep taking increasingly effective action.
Regards,
Joel Almeida
= = = = = = =
Anti-microbial treatment of LLp HD (polar lepromatous leprosy)
Adequate anti-microbial treatment of LLp HD is important for the protection of individuals and for ending transmission. What does the evidence suggest?
Microbiological evidence
The anti-M.leprae efficacy of drugs at various frequencies has been estimated using serial dilutions of bacilli.
The anti-M.leprae efficacy of monthly rifampicin is demonstrably inferior to daily rifampicin and even to daily clofazimine. Curiously, a single dose of rifampicin achieves vastly greater efficacy against viable drug-susceptible M. leprae (over 90%) than do subsequent daily doses of rifampicin. This is probably because the initial dose of rifampicin induces metabolic changes in humans and in bacilli, respectively. (2, 3)
Figure 2. Human tissue concentration of rifampicin over time (hours) following oral doses 10, 35 or 70 mg/kg (from ref. 4, CC BY 4.0)
The expected course of bacillary subpopulations during 1982 MDT can be calculated from the measured anti-microbial efficacies.
Figure 3. Expected course of bacillary sub-populations over time (days) during monthly rifampicin + daily clofazimine + daily dapsone (1)
In a polar LL patient with anergy to M.leprae, the measured anti-microbial efficacies suggest that at least 18 months of MDT using monthly rifampicin are required to kill all viable "non-persister" bacilli. Beyond that, reinfection of genetically anergic persons (5-9) still remains a threat in endemic areas.
Patients who fail to take their daily self-administered doses of dapsone + clofazimine receive, in effect, only monthly rifampicin + one monthly dose of clofazimine (if that). A monthly dose of rifampicin has roughly the same anti-microbial efficacy as 30 doses of daily dapsone.(1) A large monthly dose of clofazimine has not apparently been tested in experiments, but is unlikely to equal 30 daily doses of clofazimine in antimicrobial efficacy. In situations where self-administration seems over-optimistic, depot injections such as acedapsone (every alternate month) could well be helpful .
Other substitutes for clofazimine or dapsone or rifampicin have good anti-microbial efficacy: rifapentine, moxifloxacin, minocycline, clarithromycin. (9-15) This allows more rapid and complete elimination of endogenous bacilli, and even some intermittency between doses. However, once discontinued no regimen protects against reinfection of persons with LLp genomes in high endemic zones. Therefore in high endemic zones use of such potent anti-microbial agents is probably best accompanied by public health campaigns that almost instantly switch off nearly all human sources of infection. The most successful approach for such "switching off" has been annual skin camps (for all skin conditions) with annual mass multi-drug administration of one dose (3 bactericidal drugs such as rifampicin + ofloxacin + minocycline). This can lead to a 90% drop in new cases among those receiving the dose, while delaying selection of drug-resistant mutants. (16)
Epidemiological evidence
The WHO demonstration project of MDT in Karigiri (India) included treatment till smear negativity of two groups of MB patients. Nearly all these patients at some point had a skin smear BI (bacillary index) of 2+ or more. One group received acedapsone injections every 2 months in addition to daily oral dapsone.(17) Acedapsone releases dapsone gradually at a mean blood level of 42ng/ml with a half-life of between 15 days and 40 days.(18) Even 75 days after the acedapsone injection the blood level remains as high as 15 ng/ml. The MIC (minimal inhibitory concentration) of M. leprae for dapsone is estimated to be as low as 3 ng/ml. Therefore effective dapsone levels were maintained in the acedapsone group whether or not the daily self-administered dapsone was taken. Further, the acedapsone group had been aided by a regular second monthly dose of rifampicin and clofazimine, given on the day following each monthly dose.
No recurrence was observed among survivors in the acedapsone group at Karigiri, during an average of 16 years follow-up after smear negativity. (17) Smear negativity marked the withdrawal of MDT. Recurrence rates may well have been decreased by the assured levels of dapsone, especially in highly bacillated anergic patients. The extra monthly dose of rifampicin/clofazimine too worked against recurrence..Further, the risk of reinfection is likely to have declined as the incidence rate of LL HD in the population declined rapidly and approached zero.
Figure 4. The incidence rate of LL HD declined to near-zero with the use of MDT till smear negativity (based on ref 19)
In the group with MDT including only self-administered oral dapsone, the risk of recurrence is calculated to be <0.2/100 person-years of follow-up. (17) Interestingly, those who died or migrated before the follow-up included disproportionately more persons with a high initial BI, or with an LL classification. Therefore the reported recurrence rates here are likely to be an underestimate of the true recurrence rate among all persons in each group.
The entire 400,000+ population served by the Schieffelin Centre in Karigiri had already experienced a rapid decline in the incidence rate of LL HD (about 16%/year).using prolonged dapsone. The switch from dapsone to MDT did not reverse this rapid decline.(19) Only after MDT was shortened (eventually to only 1 year) was the decline in incidence rate of LL and MB HD clearly lost. After that, persons with anergy had to do battle with any re-infecting bacilli but without the aid of any anti-microbial protection. This scenario was replayed in endemic countries across the world. It was the equivalent of sending soldiers into battle against a dangerous foe, but armed with only blanks instead of live ammunition. The consequences were not favourable.
Figure 6. New MB cases/year reported over time, 1985 to 2019. Decline is modest or absent.
The contrast between fixed duration MDT and prolonged MDT was evident also in Weifang/Shandong vs Wenshan/Yunnan.(Fig 7, 22) In Uele (DRC) too, when prolonged dapsone was replaced by 12-month multidrug regimens, the rapid (17%/year) earlier decline in incidence rate of MB HD was lost. (23)
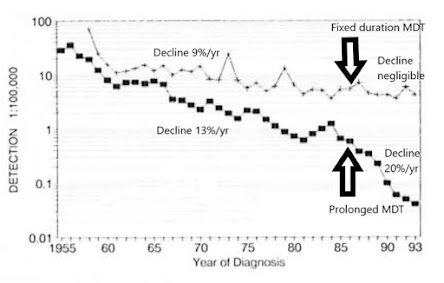
Figure 7. Detection rate of new cases in two prefectures of China. Upper line is Wenshan/Yunnan, lower line is Weifang/Shandong. (based on ref 22) Weifang/Shandong rapidly reduced transmission to near zero by ensuring prolonged anti-microbial protection for LL patients.
The practice in many endemic countries since 1998 has been to withdraw MDT from even anergic "slow responders" (mostly with polar LL genomes) after only 12 months. However, the measured anti-microbial efficacy of drugs suggests the need for a minimum of 18 months, at least in LLp patients. Anything shorter is predicted and observed to maintain transmission while needlessly allowing viable bacilli to keep inducing nerve damage. Further, premature withdrawal of MDT too often has been followed by wider neglect of previously treated LL patients. They are left, too frequently, to languish in extreme poverty and often with worsening visible deformities, excluded from the normal entitlements of civilised society including adequate anti-microbial treatment. In endemic countries, only enlightened private practitioners and centres of excellence have been providing longer anti-microbial protection to persons with LL HD.
If Karigiri's pre-1990 approach of treatment till smear negativity and accompanying 16%/year decline had been emulated across India and the world, there likely would have been a true 97% decrease in new MB patients/year between 1998 and 2019, with only 5000 new MB patients occurring globally in 2019. Instead, about 130,000 newly diagnosed MB patients were reported in 2019. About 125,000 of those are attributable at least in part to the switch from prolonged MDT to 12 month MDT for even "slow responder" anergic LLp patients (polar or "de novo" LL). A substantial proportion of these excess MB patients are likely eventually to show visible deformity.
About 1.8 million excess MB cases can be calculated as arising from the shortened duration of MDT for even "slow responder" polar LL patients since 1998. Given the frequency of nerve damage in MB HD patients during and after MDT, (21) about 500,000 of the needless new LL and BL patients are likely to have developed permanent nerve damage and visible deformity. Visible deformity entails psychological, social and economic consequences. These needless new patients have been paying a heavy price for our over-optimistic practices that are inconsistent with the measured anti-microbial efficacy of drugs. We cannot change the past, but we can change the future.
Discussion
The principle of adequate anti-microbial protection for persons with anergy is well established, and an urgent matter. In theory recurrence can be diagnosed promptly. In practice, the sequelae of previous LL disease make it very difficult to suspect or diagnose recurrence. Consequently, recurrences tend to go undiagnosed and untreated for too long. That is why prolonged anti-microbial protection is so critical for those who ever showed a high BI.
A range of options is available, taking into account adverse effects, non-ingestion of drugs, the availability of sources of infection, and other relevant factors. But options readily can be tailored to the context of each area. Only one option falls short of normal scientific standards.
Denying highly bacillated anergic persons anti-microbial protection not only exposes them to escalating permanent damage exacerbated by viable bacilli, but also forces them, following reinfection, involuntarily to keep shedding astronomical numbers of highly concentrated viable bacilli. (24) This seems not entirely consistent with science, ethics, or article 25.1 of the Universal Declaration of Human Rights.
Figure 8. Epidemiology of HD for ending transmission. The reproductive number R0 of LL HD is the critical parameter. Once this is reduced to less than 1, HD declines reasonably rapidly. Prolonged anti-microbial protection for persons with genetically determined anergy is not only ethically sound, but also important for ending transmission.
Conclusion
Since 1998 the duration of anti-microbial protection for persons with LL HD has been inadequate. The consequences were not intended by anyone: M. leprae have been spared and humans have been disfigured. Wouldn't it be good to achieve the opposite, to spare humans and damage bacilli instead? Wouldn't it be good also if relatively rapid declines of HD incidence were achieved more widely, to match the successes of Shandong, Uele and Karigiri (16% to 20%/year decline in incidence rate of MB and LL HD)? Let's make such success happen everywhere, especially in low-income high-endemic areas.
We could use an even more vigorous approach in hyperendemic hot spots, to boost the annual decline in incidence rate far beyond the 16% to 20% demonstrably available with prolonged MDT for LL patients. That more vigorous approach would use annual skin camps + an annual dose of mass ROM (or similar multi-drug prophylaxis that delays selection of drug-resistant mutants). A prompt 90% annual decline in incidence rate is achievable with this more vigorous package of interventions applied annually.
Only the most effective interventions are good enough for the esteemed patients and people we seek to serve.
References
1. Almeida, JG. A Quantitative Basis for Sustainable Anti-Mycobacterium leprae Chemotherapy in Leprosy Control Programs. Int J Lepr (1992) 60(2):255-268
2. Holdiness MR. Clinical pharmacokinetics of the antituberculosis drugs Clin Pharmacokinet 1984;9(6):511-44. doi: 10.2165/00003088-198409060-00003.
3. Zhu JH, Wang BW, Pan M et al. Rifampicin can induce antibiotic tolerance in mycobacteria via paradoxical changes in rpoB transcription. Nature Communications 2018; 9: 4218.Published online 2018 Oct 11. doi: 10.1038/s41467-018-06667-3.
4. Aljayyoussi, G.; Donnellan, S.; Ward, S.A.; Biagini, G.A. Intracellular PD Modelling (PDi) for the Prediction of Clinical Activity of Increased Rifampicin Dosing. Pharmaceutics 2019;11: 278
5. Gaschignard J, Grant AV, Thuc NV, Orlova M, Cobat A, Huong NT, et al. Pauci- and Multibacillary Leprosy: Two Distinct, Genetically Neglected Diseases. PLoS Negl Trop Dis 2016; 10(5): e0004345. https://doi.org/10.1371/journal.pntd.0004345
6. Chakravarti MR, Vogel F. A twin study on leprosy Georg Thieme Publishers, Stuttgart, Germany; 1973.
7. Cambri G, Mira MT. Genetic Susceptibility to Leprosy—From Classic Immune-Related Candidate Genes to Hypothesis-Free, Whole Genome Approaches. Front. Immunol., 20 July 2018 | https://doi.org/10.3389/fimmu.2018.01674
8. Sartori PVU, Penna GO, Bührer-Sékula S et al. Human Genetic Susceptibility of Leprosy Recurrence. Scientific Reports 2020; 10: Article number: 1284
9. Gelber RH. Activity of minocycline in Mycobacterium leprae-infected mice. J Infect Dis. 1987 Jul;156(1):236–239.
10. Ji B, Jamet P, Perani EG, Bobin P, Grosset JH, Ji B, et al. Powerful bactericidal activities of clarithromycin and minocycline against Mycobacterium leprae in Lepromatous leprosy. J Infect Dis. 1993;168:188–190. doi: 10.1093/infdis/168.1.188.
11. Gelber RH, Murray LP, Siu P, Tsang M, Rea TH. Efficacy of minocycline in single dose and at 100 mg twice daily for lepromatous leprosy. IntJLeprOther MycobactDis. 1994;62:568–573
12. Xiong JH, Ji B, Perani EG, Pétinon C, Grosset JH. Further study of the effectiveness of single doses of clarithromycin and minocycline against Mycobacterium leprae in mice. Int J Lepr Other Mycobact Dis. 1994;62:37–42.
13. Ji B, Sow S, Perani E, Lienhardt C, Diderot V, Grosset J. Bactericidal activity of a single-dose combination of ofloxacin plus minocycline, with or without rifampin, against Mycobacterium leprae in mice and in lepromatous patients. Antimicrob Agents Chemother. 1998;42:1115–1120.
14. Consigny S, Bentoucha A, Bonnafous P, Grosset J, Ji B. Bactericidal activities of HMR 3647, moxifloxacin, and rifapentine against Mycobacterium leprae in mice. Antimicrob Agents Chemother. 2000;44:2919–2921. doi: 10.1128/AAC.44.10.2919-2921.2000.
15. Pardillo FEF, Burgos J, Fajardo TT, Dela Cruz E, Abalos RM, Paredes RMD, et al. Powerful bactericidal activity of moxifloxacin in human leprosy. Antimicrob Agents Chemother. 2008;52:3113–3117. doi: 10.1128/AAC.01162-07.
16. WORKSHOP ON THE PREVENTION OF LEPROSY, POHNPEI, FEDERATED STATES OF MICRONESIA. 25-27 MAY 1999 sponsored by the Sasakawa Memorial Health Foundation Tokyo, Japan and the Western Pacific Regional Office of the World Health Organization. Int J Lepr, 67 (4) (SUPPLEMENT)
17. Norman G, Joseph G, Richard J. Relapses in multibacillary patients treated with multi-drug therapy until smear negativity: findings after twenty years. Int J. Leprosy 2004; 72:1–7
18. George J, Balakrishnan S. Blood dapsone levels in leprosy patients treated with acedapsone Indian J Lepr Jul-Sep 1986;58(3):401-6.
19 . Norman G, Bhushanam JDRS, Samuel P. Trends in leprosy over 50 years in Gudiyatham Taluk, Vellore, Tamil Nadu. Ind J Lepr 2006. 78(2): 167-185. reviewed and analysed further in: 20a Almeida J. Karigiri, India: How transmission rapidly was reduced in a low-income population LML 29 October 2020
20. Balagon MF, Cellona RV, dela Cruz E et al. Long-Term Relapse Risk of Multibacillary Leprosy after Completion of 2 Years of Multiple Drug Therapy (WHO-MDT) in Cebu, Philippines. American Journal of Tropical Medicine and Hygiene, 2009; 81, 5: 895-9. reviewed and analysed further in 19a. Almeida J Recurrence rate among MB patients following RFT. LML 2 June 2019.
21 Croft RP, Nicholls PG, Steyerberg EW et al. A clinical prediction rule for nerve function impairment in leprosy patients-revisited after 5 years of follow-up. Lepr Rev 2003 Mar;74(1):35-41.
22. Li HY, Weng XM, Li T et al. Long-Term Effect of Leprosy Control in Two Prefectures of China, 1955-1993. Int J Lepr Other Mycobact Dis. 1995 Jun;63(2):213-221. reviewed & analysed further in: 22a. Almeida J. What really happened in Shandong? LML 16 Nov 2019
23. Tonglet R, Pattyn SR, Nsansi BN et al. The reduction of the leprosy endemicity in northeastern Zaire 1975/1989 J.Eur J Epidemiol. 1990 Dec;6(4):404-6 reviewed in: 23a. Almeida J. Reducing transmission in poor hyperendemic areas - evidence from Uele (DRC). LML 29 Nov 2019
24 Davey TF, Rees RJ. The nasal dicharge in leprosy: clinical and bacteriological aspects. Lepr Rev. 1974 Jun;45(2):121-34.
LML - S Deepak, B Naafs, S Noto and P Schreuder
LML blog link: http://leprosymailinglist.blogspot.it/
Contact: Dr Pieter Schreuder << editorlml@gmail.com







No comments:
Post a Comment